-
Site-Dependent Excited-State Dynamics of a Fluorescent Probe Bound to Avidin and Streptavidin
A. Fürstenberg, O. Kel, J. Gradinaru, T.R. Ward, D. Emery, G. Bollot, J. Mareda and E. Vauthey
ChemPhysChem, 10 (9-10) (2009), p1517-1532


DOI:10.1002/cphc.200900132 | unige:3554 | Abstract | Article HTML | Article PDF
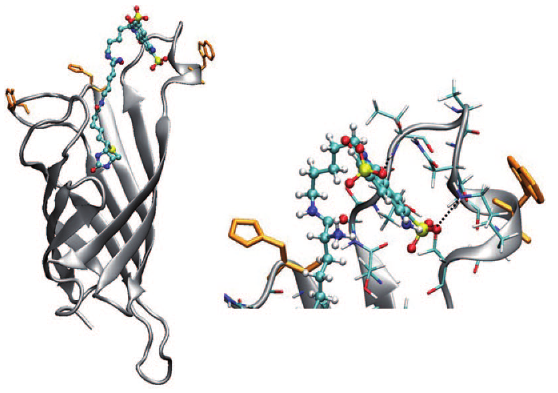
The excited-state dynamics of biotin–spacer–Lucifer-Yellow (LY)constructs bound to avidin (Avi) and streptavidin (Sav) was investigatedusing femtosecond spectroscopy. Two different locations in the proteins,identified by molecular dynamics simulations of Sav, namely the entrance of the binding pocket andthe protein surface, were probed by varying the length of thespacer. A reduction of the excited-state lifetime, stronger inSav than in Avi, was observed with the long spacer construct.Transient absorption measurements show that this effect originatesfrom an electron transfer quenching of LY, most probablyby a nearby tryptophan residue. The local environment of theLY chromophore could be probed by measuring the time-dependent polarisation anisotropy and Stokes shift of the fluorescence. Substantial differences in both dynamics were observed.The fluorescence anisotropy decays analysed by using thewobbling-in-a-cone model reveal a much more constrained environment of the chromophore with the short spacer. Moreover, the dynamic Stokes shift is multiphasic in all cases, with a~ 1 ps component that can be ascribed to diffusive motion ofbulk-like water molecules, and with slower components withtime constants varying not only with the spacer, but with theprotein as well. These slow components, which depend strongly on the local environment of the probe, are ascribed to themotion of the hydration layer coupled to the conformationaldynamics of the protein.